
Avoid or Use Alternate Drug.Įrgoloid mesylates, dextroamphetamine. Additive pressor effect.ĭoxepin, dextroamphetamine. Additive vasospasm risk of hypertension.ĭoxapram increases effects of dextroamphetamine by pharmacodynamic synergism. Additive vasospasm risk of hypertension.ĭihydroergotamine intranasal, dextroamphetamine. Avoid or Use Alternate Drug.ĭihydroergotamine, dextroamphetamine. Avoid or Use Alternate Drug.ĭextroamphetamine and desvenlafaxine both increase serotonin levels.
Risk of V tach, HTN.ĭesipramine, dextroamphetamine. Avoid or Use Alternate Drug.ĭesflurane increases toxicity of dextroamphetamine by Mechanism: unknown. Additive vasospasm risk of hypertension.Ĭlomipramine, dextroamphetamine. Avoid or Use Alternate Drug.Ĭabergoline, dextroamphetamine. Avoid or Use Alternate Drug.Ĭomment: Tricyclic antidepressants increase or decrease effects of sympathomimetics, by blocking reuptake of NE, or blocking uptake of indirect sympathomimetics into the adrenergic neuron.Īmoxapine, dextroamphetamine. Risk of acute hypertensive episode.Īmitriptyline, dextroamphetamine. Tranylcypromine increases effects of dextroamphetamine by pharmacodynamic synergism. Selegiline transdermal increases effects of dextroamphetamine by pharmacodynamic synergism. Coadministration is contraindicated during or within 14 days following the administration of MAOIs. Amphetamines should not be administered during or within 14 days following the use of most MAOIs or drugs with MAO-inhibiting activity selegiline increases effects of dextroamphetamine by pharmacodynamic synergism. Selegiline and dextroamphetamine both increase serotonin levels. Concomitant use could result in life-threatening serotonin syndrome. Coadministration is contraindicated during or within 14 days following the administration of MAOIs.Įither increases toxicity of the other by serotonin levels. Rasagiline increases effects of dextroamphetamine by pharmacodynamic synergism. Procarbazine increases effects of dextroamphetamine by pharmacodynamic synergism. Phenelzine increases effects of dextroamphetamine by pharmacodynamic synergism.
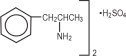
Linezolid increases effects of dextroamphetamine by pharmacodynamic synergism. Isocarboxazid increases effects of dextroamphetamine by pharmacodynamic synergism. If clinically appropriate, discontinue drugs that compete for NE receptor sites for at least 5 half-lives may cause false-negative imaging results.

6 years: 5 mg PO qDay or q12hr may increase by 5 mg qWeek not to exceed 40 mg qDay or divided q8hr use intervals of 4-6 hr between additional doses.ESRD (GFR Severe renal impairment (GFR 15 to No more than 60 mg given qDay or divided doses with intervals of 4-6 hr between doses.5-60 mg PO qDay may increase by 10 mg/day qWeek.Note: 25 mg qAM may be considered as an initial dose for some patients.May increase by increments of 12.5 mg/week not to exceed 50 mg/day.May increase by increments of 5-10 mg/week not to exceed 60 mg/day.20 mg PO qAM initially or switching from another medication.Tablet: 5 mg PO qDay initially may increase by 5-10 mg/day qWeek administer daily dose in 2-3 doses not to exceed 40 mg/day Extended-release capsule Each tab/cap contains equal portions of the following: amphetamine aspartate, amphetamine sulfate, dextroamphetamine saccharate, and dextroamphetamine sulfate tablet: Schedule II


 0 kommentar(er)
0 kommentar(er)
